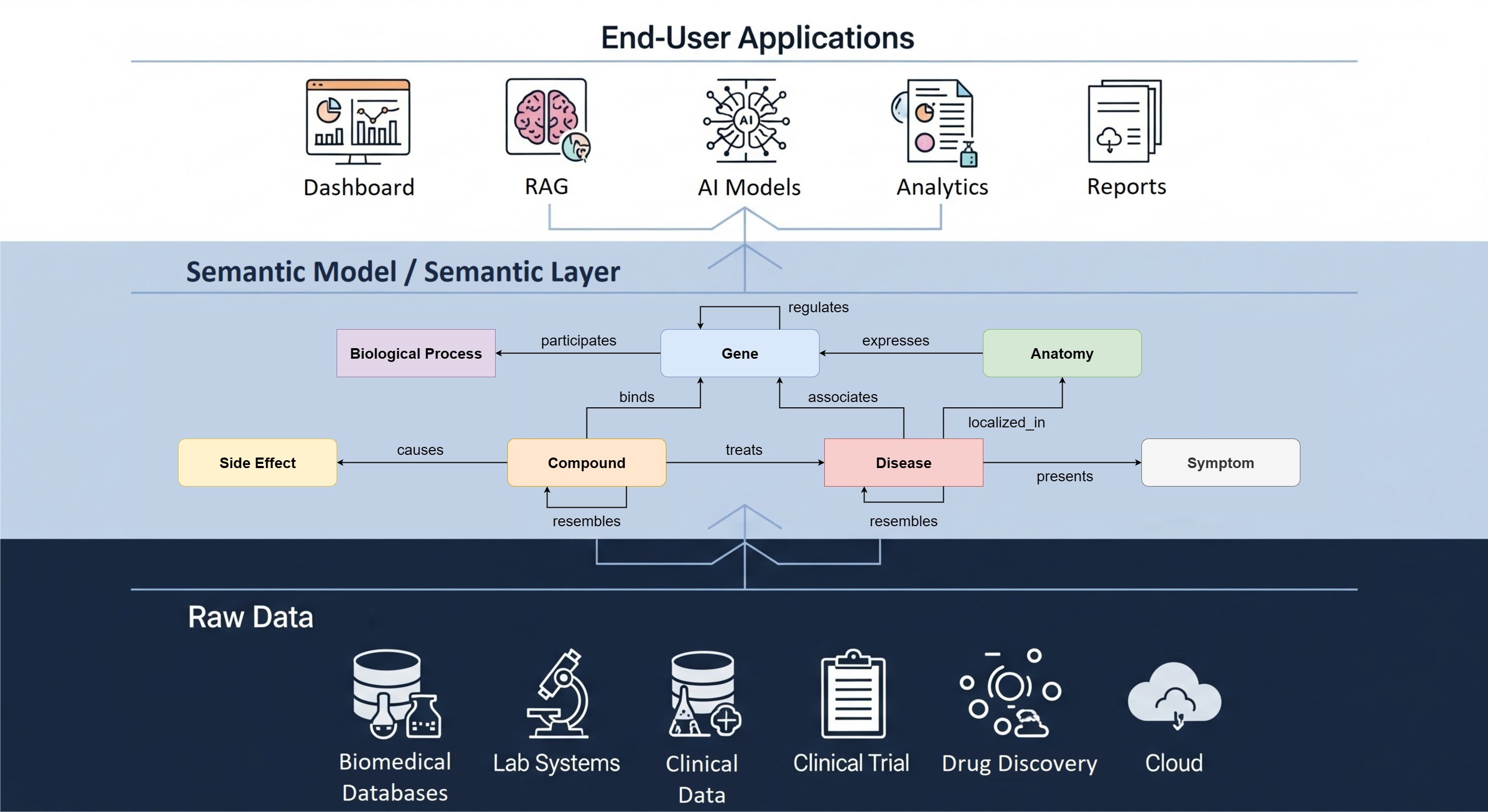
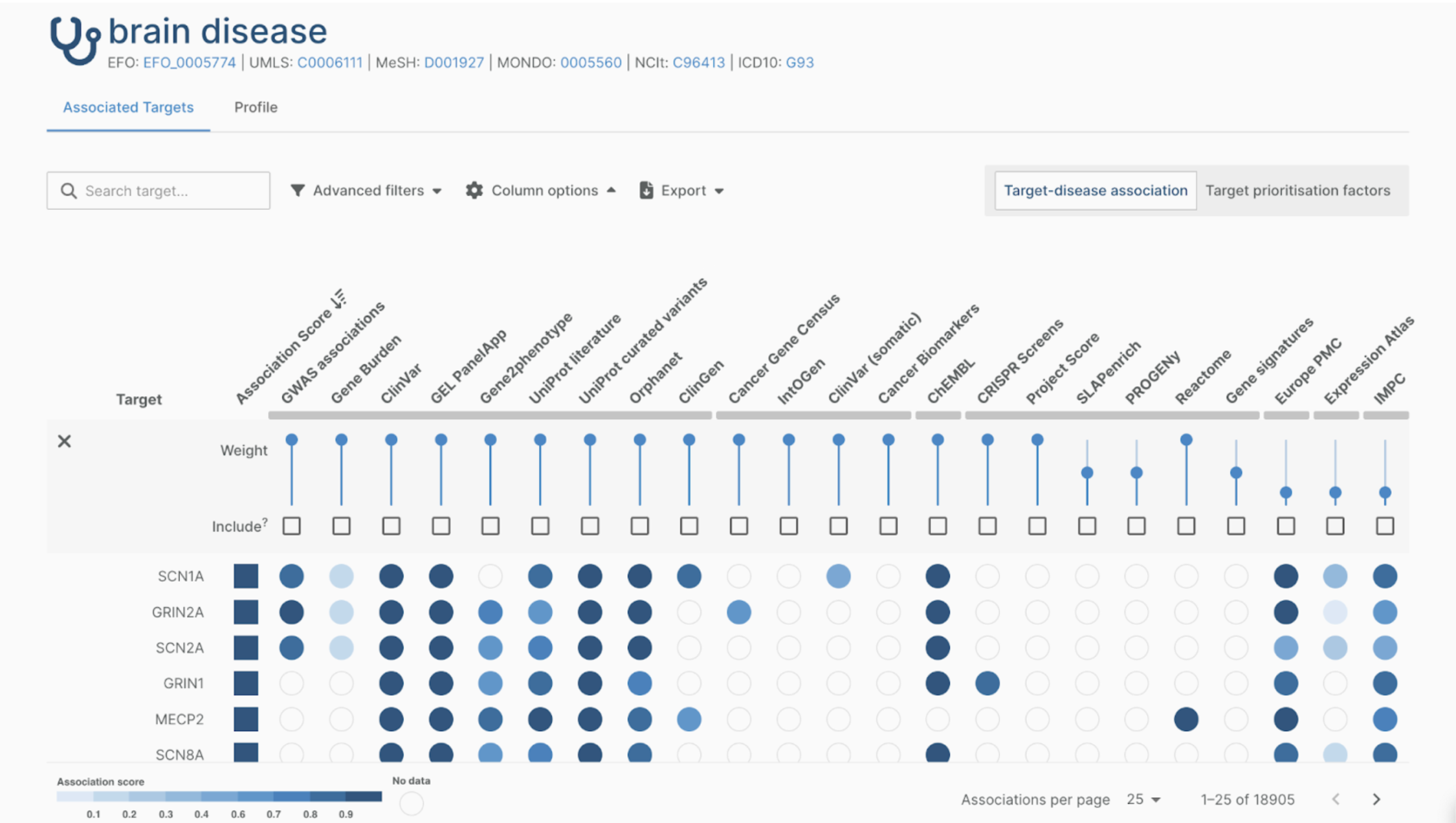
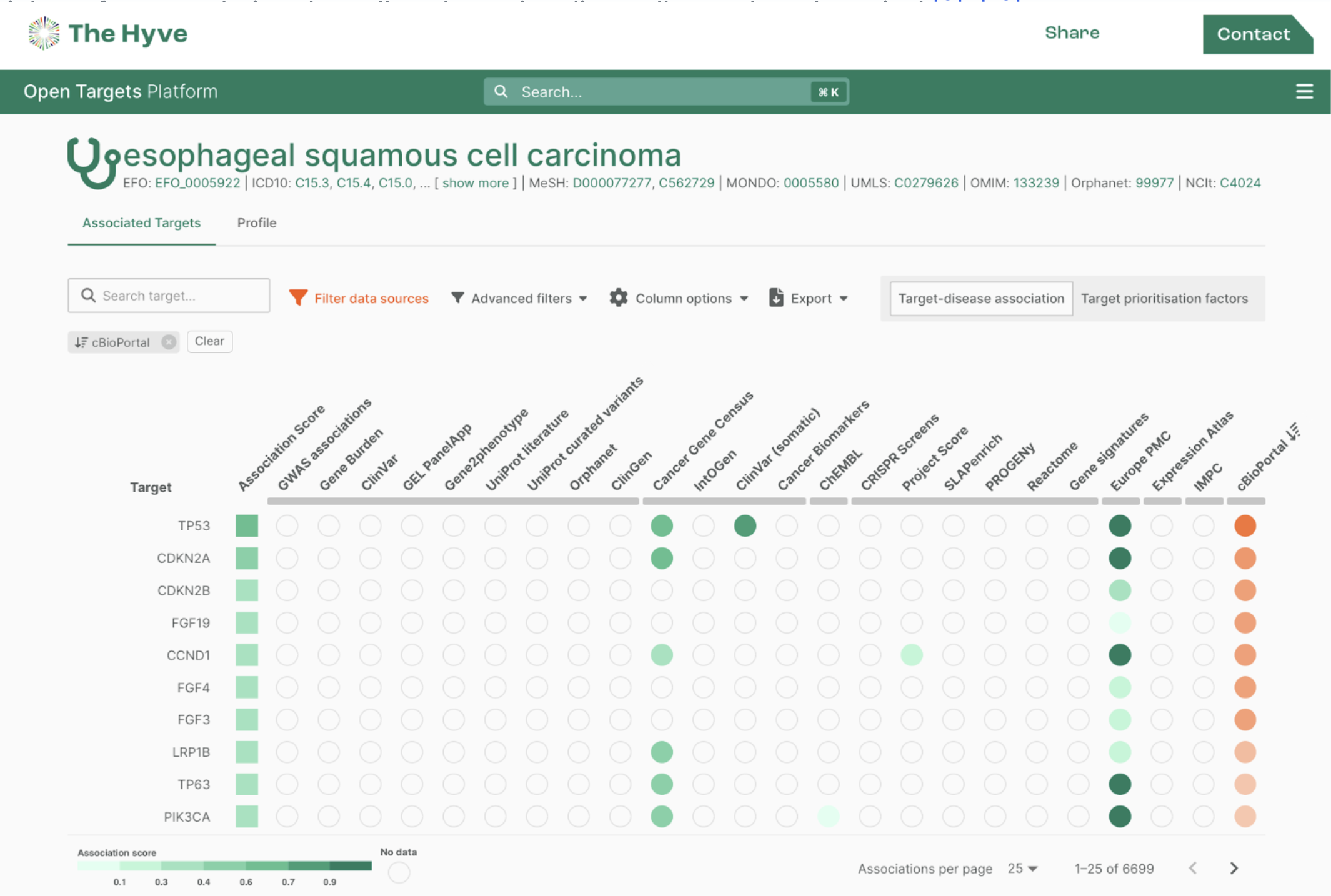
Are custom data fields or custom data models necessary?
Depending on the analytics use cases and data quality requirements, The Hyve team has substantial experience in providing consultancy and technical solutions for implementing custom fields and custom model layers. These solutions are tailored to be fit for purpose, enabling novel analytics and evidence generation.
What is Delphyne?
Delphyne is a Python package designed to simplify and standardize the process of converting source data to the OMOP Common Data Model (CDM). It offers an easy setup for ETL development projects, flexible source data loading and transformation implementation, quick mapping of source values to standard OMOP concepts, and efficient data extraction and loading options e.g. cashing. Delphyne also includes a ready-to-use ETL framework (Delphyne-template), ensuring a cost-effective and streamlined ETL development process.
Does The Hyve need to get access to the source data?
The decision to grant The Hyve access to the source data is optional, not mandatory. We understand the importance of maintaining the security of your data. As an ISO 27001-certified company, The Hyve prioritizes the protection of sensitive information. Our expertise allows us to work either with the source data or via development of an intermediary totally privacy preserving synthetic dataset used for ETL development purposes only. In summary, we respect your preferences and are here to collaborate in a way that aligns with your data security requirements.