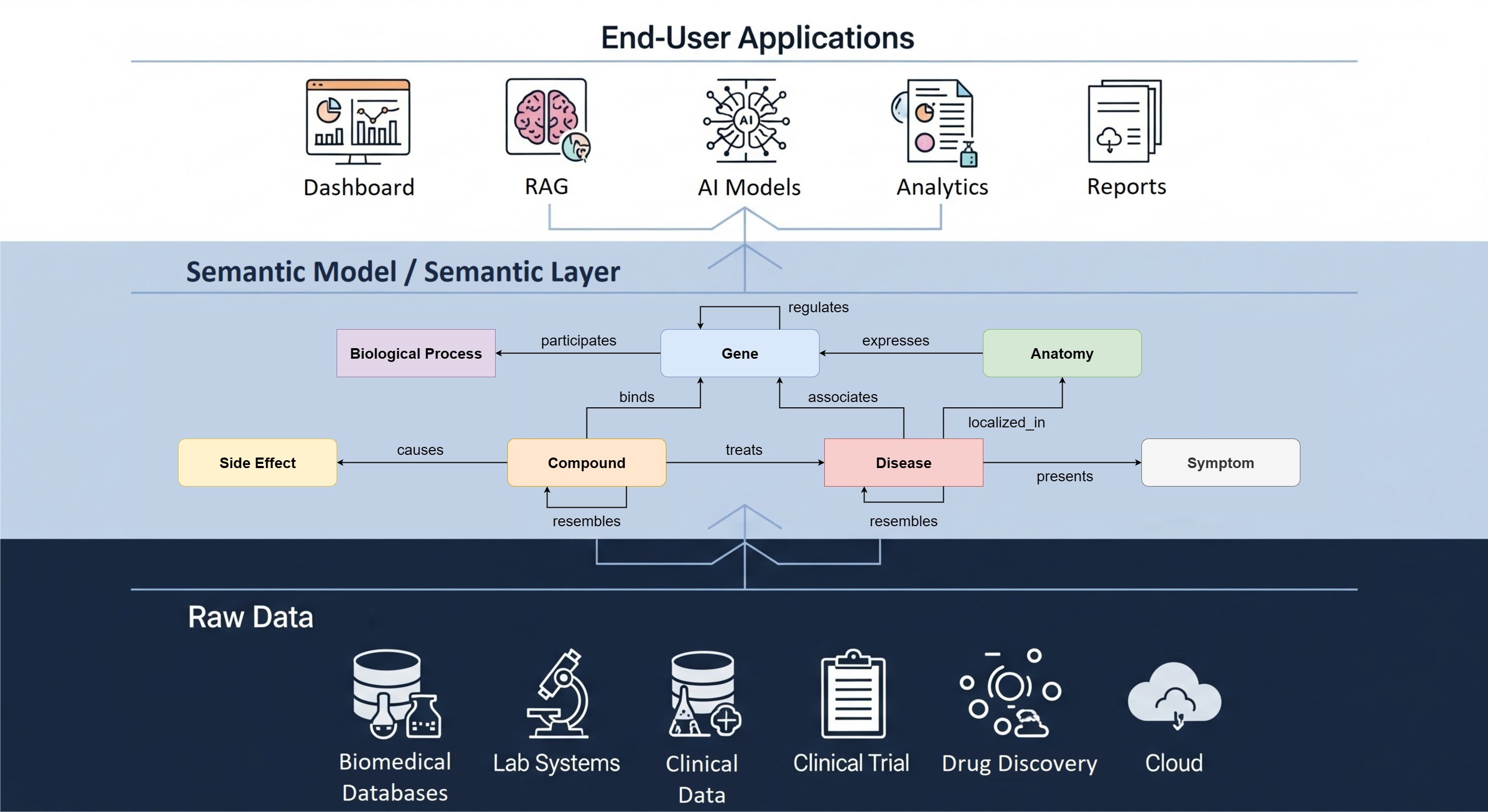
Much as its name suggests, the Observational Medical Outcomes Partnership Common Data Model (OMOP CDM) was developed with the aim of bringing together disparate observational databases to allow for a common analysis. Since its inception in 2008, OMOP has thrived in applications around Real World Data (RWD) such as, for example, harmonizing Electronic Medical Records (EMR) with administrative claims, allowing its users to conduct analyses across a wide range of data sources.
Despite OMOP traditionally being an observational model, there has recently been a growing interest in adapting the model for interventional trial data. Conforming clinical trial and observational data into one model would bring about a number of benefits, such as enabling RWD assets to support interventional analyses (more on this later).
Observational data and clinical data – what’s the difference?

At this point, it might be useful to clarify the main differences between clinical trial data and observational data. Observational data, such as EMR, is collected by healthcare facilities. These are usually accustomed to standardising their data to a common vocabulary in such a way that reimbursement for treatments can occur. Most diagnosed conditions or administered drugs have existing mappings in, or to, vocabularies like SNOMED and RxNorm. Clinical trial data on the other hand is less standardised. Trials often explore novel biomarkers and treatments, which are rarely found in common, global vocabularies. Clinical trials are also less likely to have mapped their data to a vocabulary, often representing information as free text rather than codes.
Advantages of an interventional OMOP model
For these and other reasons, representing clinical trial data in OMOP is not a straightforward process and involves some creative problem solving. So the first question to answer: Is it worth the effort? We briefly touched on one advantage of conformance, but let’s take a deeper dive into OMOP to really understand its value.

Thanks in part to its accessibility –OMOP is open source–, the OMOP Common Data Model has been widely adopted and has growing support world-wide. For example, the European Health Data and Evidence Network (EHDEN) project has chosen OMOP as their Common Data Model, and further implementations can be seen around the globe, from South Korea to the United States.
One of the reasons for its success is that OMOP is not just an open source data model, it is also a community. The Observational Health Data Sciences and Informatics (OHDSI) community has been heavily involved in OMOP’s evolution and spread. Besides, the network is teeming with collaborative efforts to constantly improve OMOP and to support its implementers and users.
On top of OMOP, there is a range of open source tooling available. Tools like Atlas allow users to analyse cohorts of patients. If interventional data were present in an OMOP CDM, users would be able to ask the same queries to both their observational data and their clinical trial data. Users could even leverage their RWD assets to create virtual control groups based on the current standard of care against which to compare the efficacy of novel treatments.

How to put this into practice?
Representing interventional data in OMOP poses some challenges. For example, how do we set up global cross-institutional conventions for mapping novel biomarkers? Where do we store all the information regarding study arms, planned visit dates and blood tests? Together with the Clinical Trials Working Group within OHDSI, The Hyve has been working to answer these and other questions with a proposed OMOP model for clinical trials.
Stay tuned for updates about this exciting work!