Who is it for?
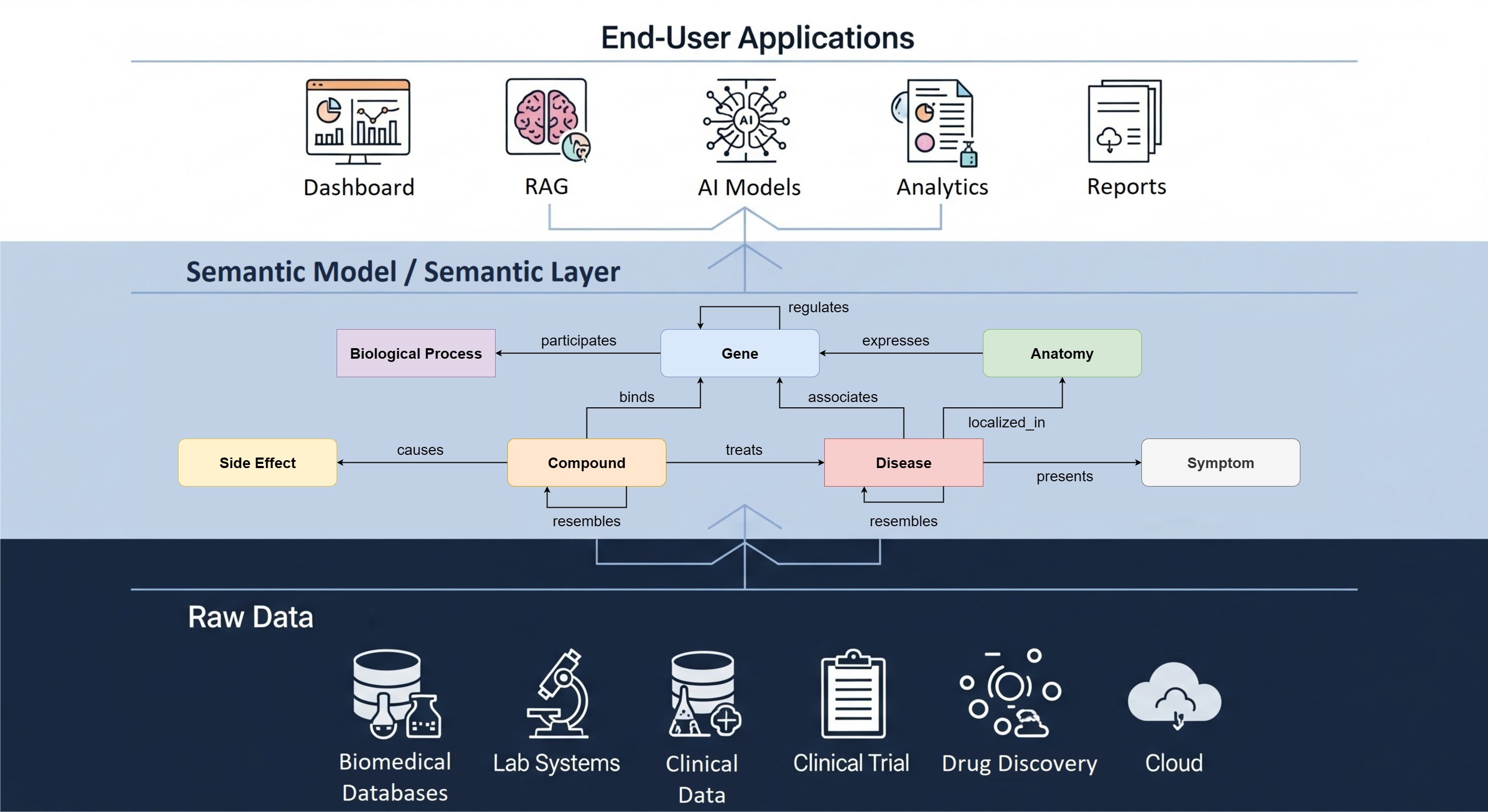
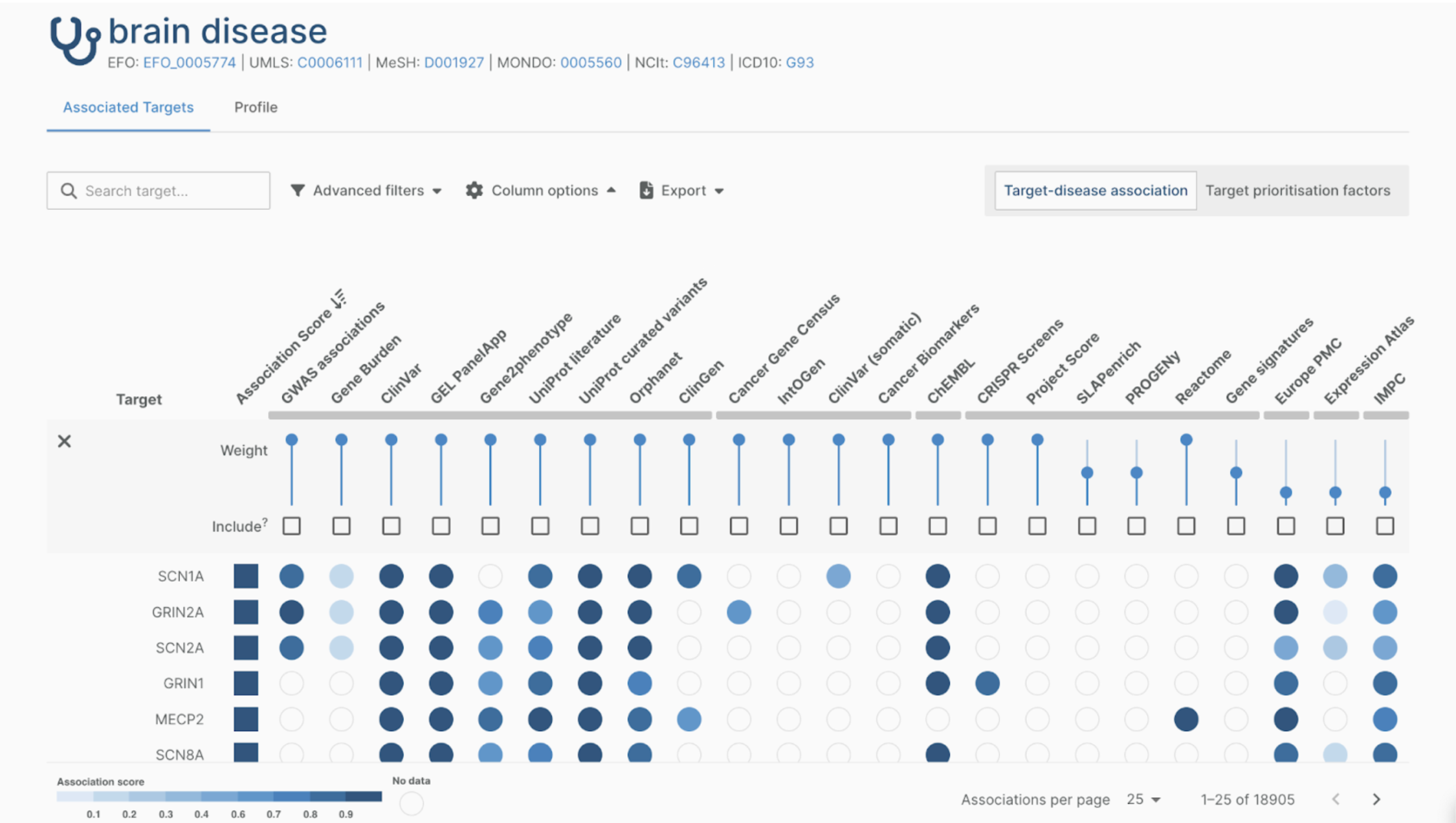
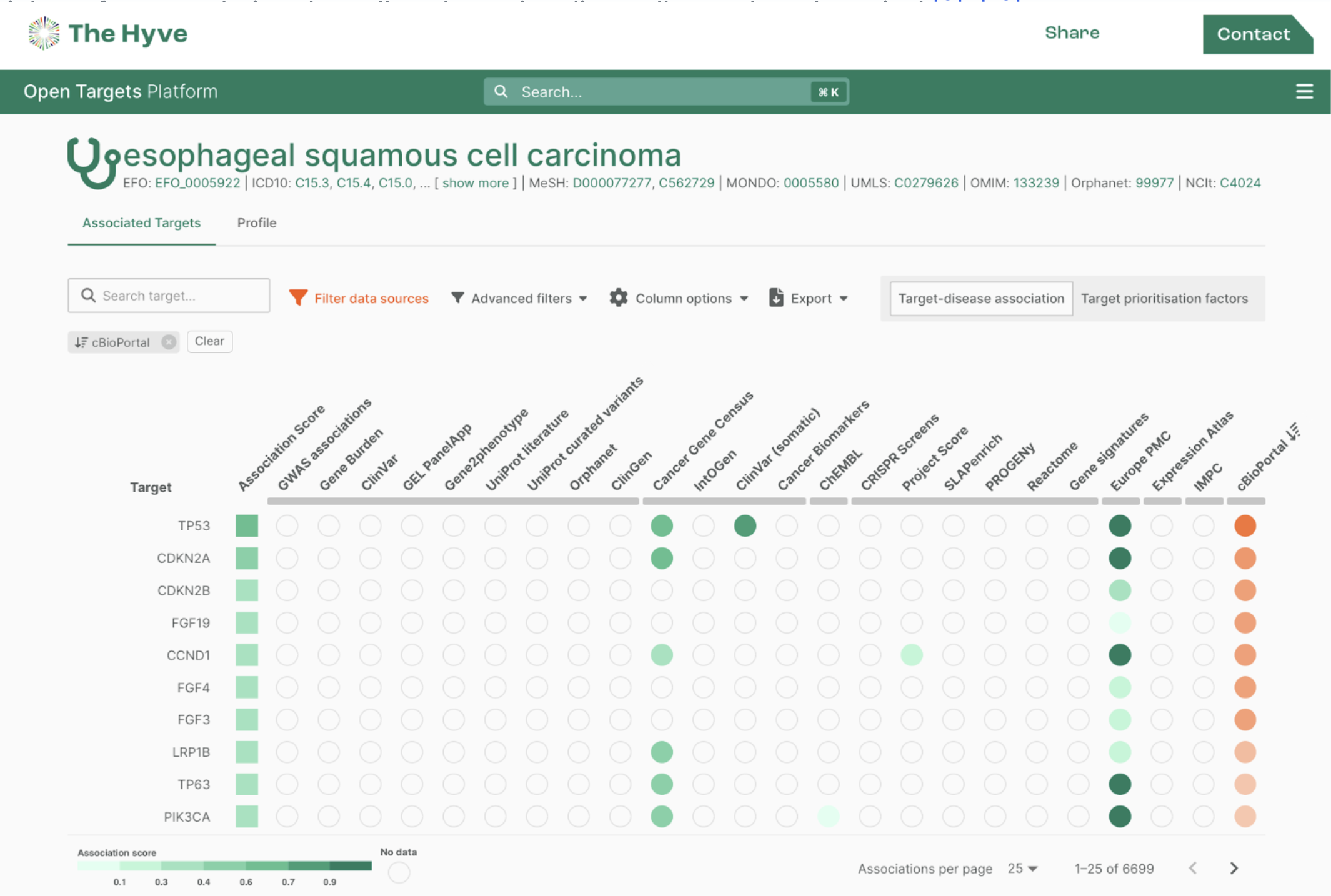
RADAR-base was designed and built to be a flexible and scalable digital health data collection platform using state of the art technologies. It is disease and device-agnostic and can thus be fine-tuned to fit a broad range of use cases. At the same time, this versatility introduces complexity and a potentially steep learning curve for newcomers. There is a wealth of existing documentation on how to use the open-source platform. However, getting to grips with these technologies can be time-consuming, and consequently expensive.
RADAR-base as a Service package was designed specifically to support those in need of a quick start to RADAR-base. Our service introduces you to the platform, including all its core features and helps you understand the different modalities and the quality of active and passive data collected by wearable devices and mobile applications. Since we set up and deploy the platform, you don't need to invest in or depend on in-house technical and IT resources for use of RADAR-base.